

Batch control identification is a mandatory requirement when sterilising critical items that are used between patients. This is a legal requirement in the event a patient makes claims about inadequate instrument reprocessing in the practice. Together with batch control of instruments we must also keep our steriliser cycle record book. This is best kept in hard copy.
There are various ways instrument packs can be labelled for tracking – through handwriting information on the packs (with correct pens), using a manual labelling gun with adhesive labels or with labels printed by an electronic barcoding system. We must record key information like the steriliser number, the cycle number, the date of processing and the initials of the person responsible. 
Some facilities may have electronic systems in place however, many facilities still use manual procedures where hard copy patient record charts are updated and filed or scanned electronically into the system.
Computer printed documentation labels with barcode only link the contents of a load with a sterilisation cycle.
We have to go back to other means of cycle verification to prove that a pack went through a steam sterilisation process. While electronic cycle reports or physical chart print outs verify that cycle parameters were achieved, it is also mandatory to use a Type 1 chemical indicator on all packs as visual proof each pack was exposed to a steam sterilisation process.
This is why steri-peel packs have a Type 1 indicator on them. But we know once that pack is opened and the instruments retrieved, that pack is going in the bin.
So how do we verify later that instruments that were used on a patient have indeed been through a steam sterilisation process? This is of particular importance when using critical instruments between patients and it is a mandatory requirement to have a system in place to track these items.
Whether you are using an electronic system or a manual documentation system – using the GKE Documentation Labels with Type 1 indicator offers you the following key benefits:
1. Instant clear visual verification of a process having taken place. A pack should go through various checks for having been processed, including a final check at chairside before the pack is opened. The GKE labels give clear assurance at chairside.
2. Ability to remove the processed label from the pack and adhere to the patient’s chart.
Whether record keeping is physical or electronic, the patient’s notes with GKE indicator adhered, can be maintained as proof that a pack went through a steam sterilisation process.
3. The GKE indicators can be maintained for years, and the chemical indicator colour is guaranteed to not revert or change in any way.
4. Four label colours to choose from (green, blue, red and yellow) all with clear precise colour change of chemical indicator from blue to black. You might choose to use a different colour to denote a different steriliser, a different member of staff or to categorise your instrument packs.
The GKE Manual Documentation System
The GKE System comprises the labelling gun, rolls of labels and logbook.
While we have our Type 1 indicators indicating a package has been through a process, we know that the indicator gives no information about sterility. All it tells us is that the package has been exposed to steam.
This is where we should consider the use of additional chemical indicators.
A PCD should be considered for routine monitoring of all loads, or at least loads with critical instruments or implants at a minimum. PCD’s give us information that no other chemical indicator can. They simulate our instruments with hollow cavities (like handpieces) and tell us about whether air removal and steam penetration have taken place. This principle is very simple for porous items (cotton, gauze) or solid items (like mirrors, explorers or forceps), but is more challenging for more complex instruments with hollow cavities (like hand pieces and burs).
Use of a PCD assures us that air removal and steam penetration occurred for both external and internal surfaces of all instruments.
The GKE Orange PCD has the dual benefit of being able to be used for your Daily Air Removal and Steam Penetration Test as well as for routine monitoring of all loads.
And is guaranteed to last in excess of 10,000 cycles when handled in accordance with the DFU’s.
This device means less plastic waste – so its environmentally friendly!
The GKE PCD indicator can be adhered to cycle records maintained in the hardcopy steriliser cycle record book.
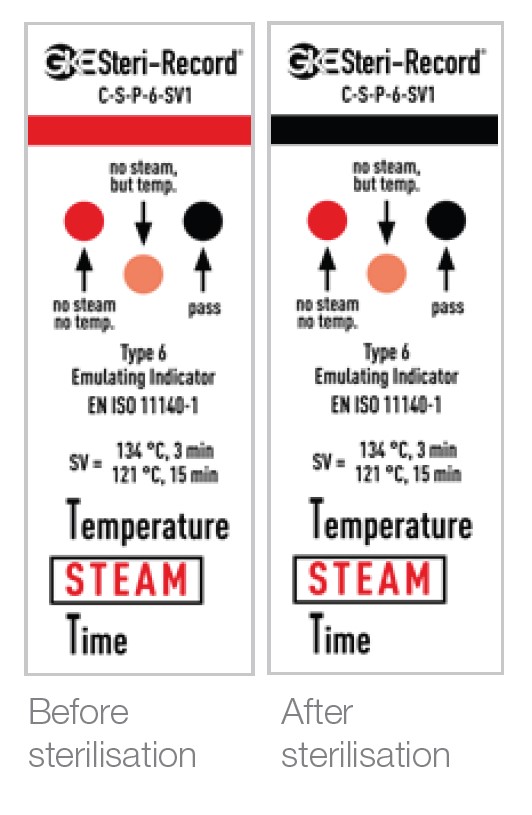
2. Other Package Monitoring Indicators, eg GKE Type 6
While not mandatory for use if you have a cycle report/chart print out, consideration should be given to the use of these indicators inside instrument packs. These indicators give information about their point of placement and indicate that sterilisation conditions have been met in that position. (Note: they can’t give information about the insides of hollow instruments like a PCD can).
A Type 6 indicator is often recommended as it reacts to all critical variables, i.e. temperature, time and steam.
The benefit of the GKE indicators is the self-adhesive backing that can be adhered to your patient’s notes (together with the Type 1 indicator) or other applicable documentation kept by the practice.
It’s about Best Practice
Aside from the mandatory requirements, each practice must consider their own risk protocols and what additional processes and procedures are appropriate for them, while also considering what is best practice. Because best practice means we are doing what’s best for the patient.

Article first published in May/Jun 2022 Dental Solutions